Which Term Best Describes All Atoms in Ionic Bonds
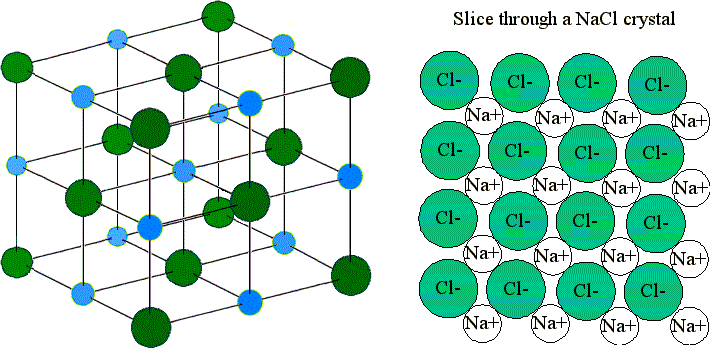
These lattices are not called molecules. The cation are positively charged and an anions are negatively charged.

Ionic Bond Definition Properties Examples Facts Britannica
Electrovalent bonds are produced when electrons are transferred from atoms of one element to atoms of another element producing positive and negative ions.

. The correct option is A Stable. Molecule is the general term used to describe any atoms that are connected by chemical bonds. White gold is considered an alloy of nickel and gold.
Is the only element in group 1 on the periodic table that forms covalent bonds. All compounds are molecules but not all molecules are compounds. Atoms with large differences in electronegativity transfer electrons to form ions.
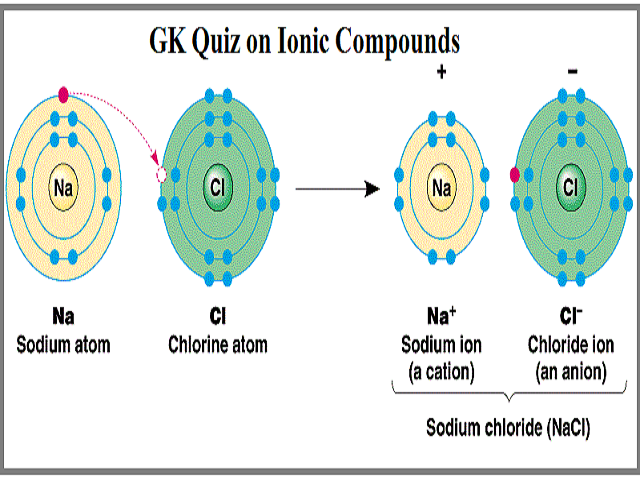
Electrons are transferred from the chlorine atom to the lithium atom. One atom becomes more electronegative than another atom. O atoms breaking bonds O ions breaking bonds water molecules breaking bonds O water molecules surrounding ions Which of the following best describes the reason ionic substances dissolve O ions break bonds O positive ions are attracted to negative ions water molecules are attracted.
Which of the following terms best describes dissociation. They conduct electricity but only when they are dissolved in water. Two atoms attain equal electronegativities.
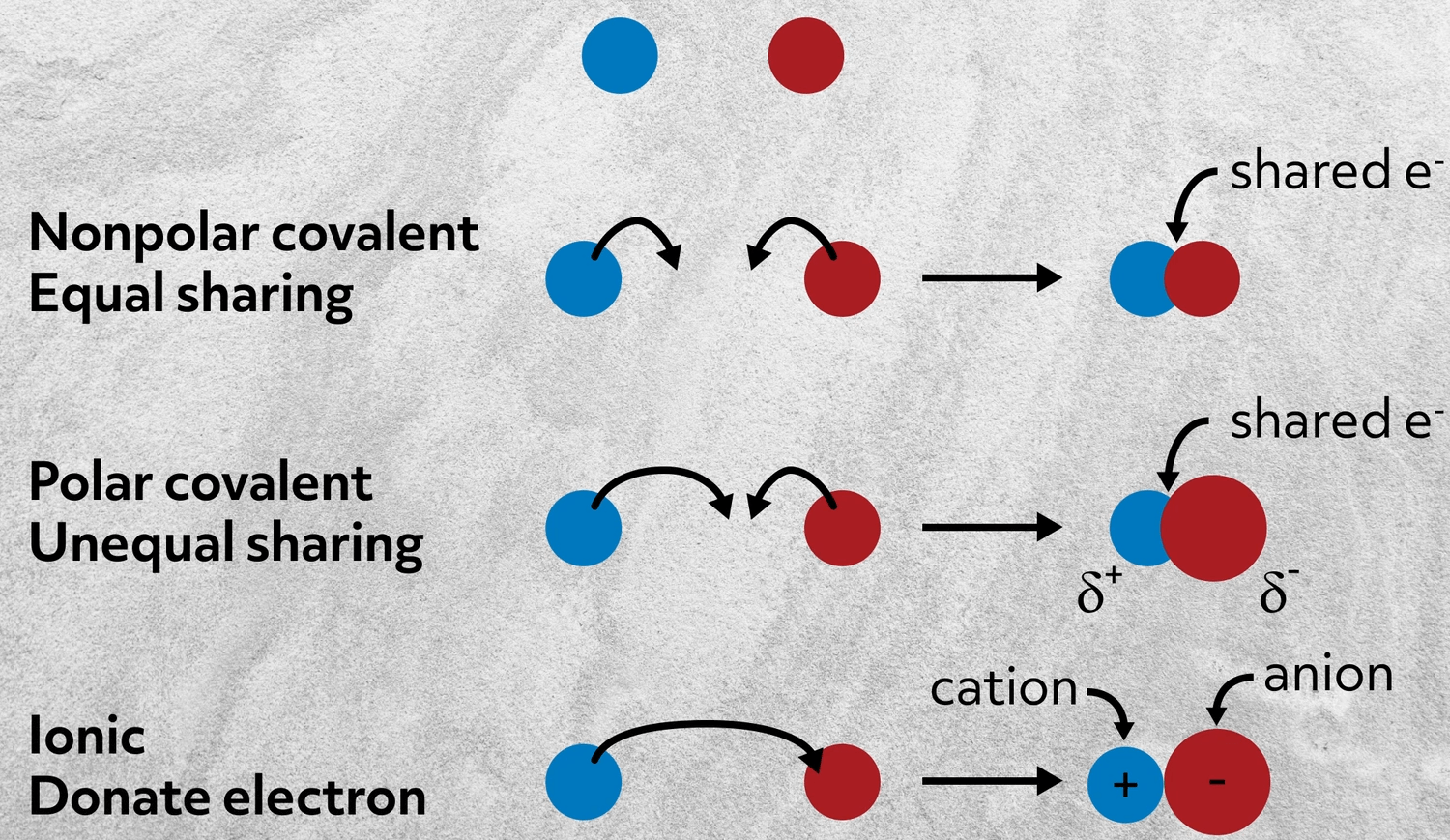
Question 27 3 points Which term best describes an equal sharing of a bonded pair of electrons between atoms non-polar covalent bond dipole moment octet rule polar covalent bond ionic bond Get the answer to your homework problem. What electrons form a ionic bond. The bond formed between any two atoms is not a purely ionic bond.
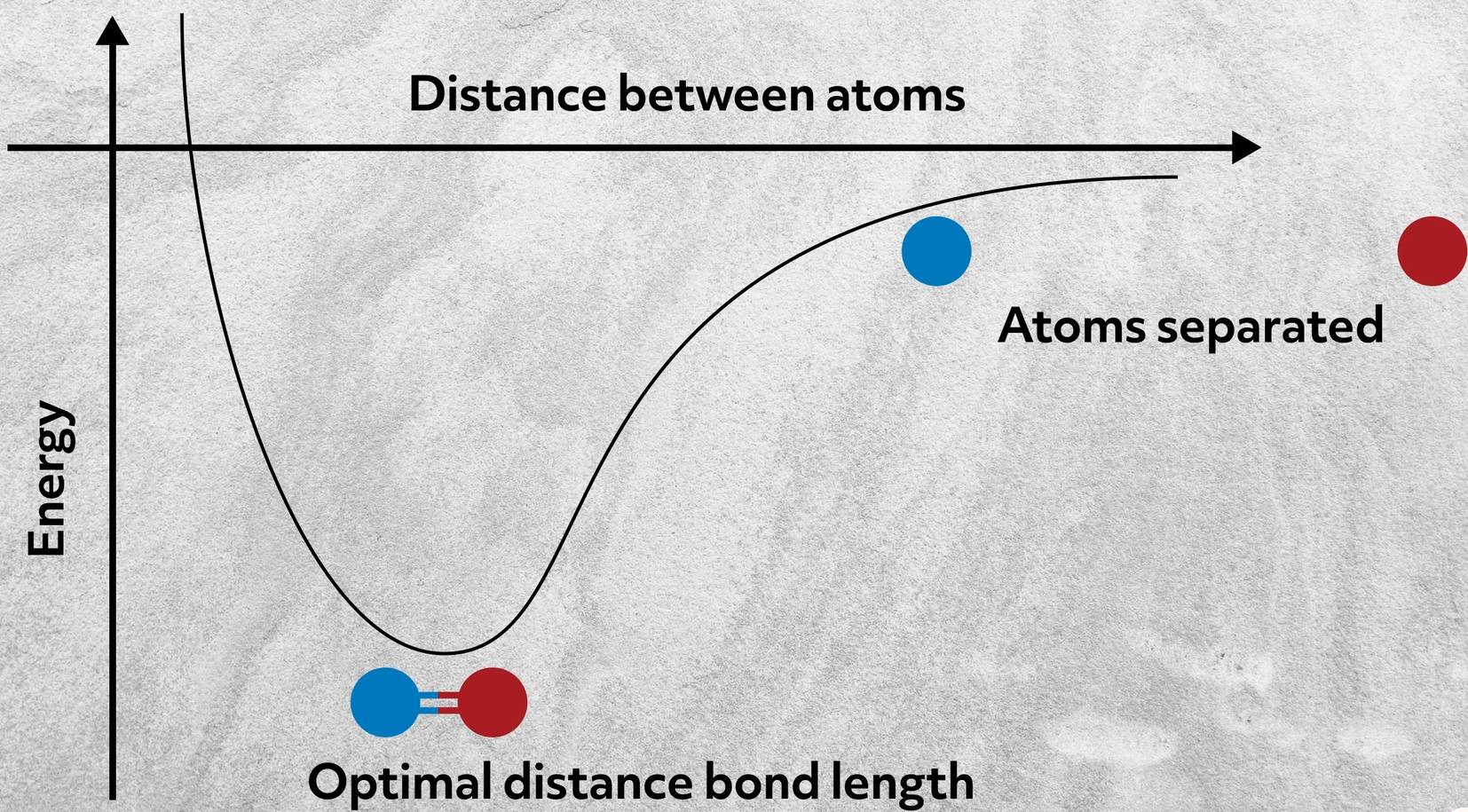
This attraction is known as an ionic bond. The carbon atom with four electrons in its outer shell is constructed so that it readily bonds covalently with four other atoms. The most useful kinematic formula would be the velocity of the motorcylce as a function of time which is.
When two pairs of electrons are shared between two atoms a bond is formed. Both metals and nonmetals lose electrons to form bonds. This type of bonding typically takes place between metal and nonmetal atoms.
They have high melting points and also high boiling points. They have higher enthalpies of fusion and vaporization than molecular compounds. The bond which is formed by the transfer of electrons between the atoms is called electrovalent bond or ionic bond.
What two atoms form ionic bonds. In a methane molecule CH4 there are 4 single covalent bonds. Which of the following terms best describes dissociation.
Cl2 contains a covalent bond. The formation of an ionic bond involves the. Two atoms will form an ionic bond by the complete transfer ofthe.
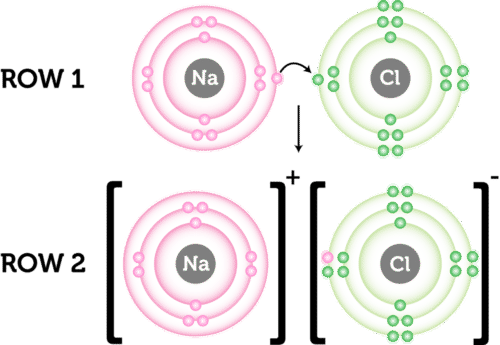
The ions then are attracted to each other. An atom of lithium Li forms an ionic bond with an atom of chlorine Cl to form lithium chloride. Which of the following best describes the way ionic bonds are formed.
Metals lose electrons and nonmetals gain electrons to form bonds. Where v_0 is the initial velocity and a is the acceleration. Two atoms of the same element will never form an ionic bond.
The number of atoms is the same in the reactants and in the products and the total mass is the same in the reactants and in the products. Every combination of atoms is a molecule. This makes large molecules possible.
All bonding interactions have some covalent character because the electron density remains shared between the atoms. How are the valence electrons of these atoms rearranged to form this bond-A few valence electrons are shared between the atoms-Many valence electrons are shared between the atoms. One atom pulls an electron from another atom.
Ionic bonds form when a nonmetal and a metal exchange electrons while covalent bonds form when electrons are shared between two nonmetals. But be aware that ionic lattices also contain more than 2 atoms that are joined by ionic bonds. Metals gain electrons and nonmetals lose electrons to form bonds.
Alloy is the term which refers to the combination of metals in which a new. An ionic bond is a type of chemical bond formed through an electrostatic attraction. Ionic compounds are the compounds which are made up of an ions that are cations and an anions.
The correct answer is Option A. Electrovalent bonds are only formed between metals and non-metals. Atoms breaking bonds O ions breaking bonds water molecules breaking bonds water molecules surrounding ions Which of the following best describes the reason ionic substances dissolve O ions break bonds O positive ions are attracted to negative ions water molecules are attracted.
Donald is writing a chemical name from a chemical formula. A non-polar covalent bond is one in which the electrons are shared equally between two atoms. Define the two terms monomer and polymer and relate them to each other.
What are 5 properties of ionic. 30 Questions Show answers. A compound is a molecule made of atoms from different elements.
A link or force between neighboring atoms in a molecule or compound. An attraction between two ions used to create an ionic compound. Which of the following happens when an ionic bond is formed.
Ionic bonds form when one atom gives one or more electrons to another atom causing them to stick together. 2013-08-09 11. Which best describes white gold.
However the problem states that the motorcyle start at rest therefore v_0 0. Both metals and nonmetals gain electrons to form bonds. The element which donates the electrons is known as electropositive element and the element which accepts the electrons is known as electronegative element.
Ionic bond is formed when there is complete transfer of electrons from one element to another element.

What Are Ionic Compounds Definition Structure Properties Examples With Videos Of Ionic Compounds Ionic Character

Lesson Worksheet Ionic Bonds Nagwa

Ionic Bonding Definition Properties Examples By Chemistry Topics Medium

Question Video Selecting The Statement That Does Not Describe Ionic Bonding Nagwa

Crystal Types Of Bonds Britannica
9 4 Ionic Bonding Chemistry Libretexts

What Is The Electronegativity Of Ionic Bonds Quora

Ionic Bonding Eve Wongworakul Chemistry Unit
What Are Ionic Covalent And Metallic Bonds Quora

The Ionic Bond Chemistry Master

Ionic Compounds Bonds Structure Properties 1 6 5 Edexcel Igcse Chemistry Revision Notes 2019 Save My Exams

Electrons Atom Charge Forces And Ionic Bounding Physics Stack Exchange
Could Two Atoms Engage In Ionic Bonding Why Or Why Not Quora

Comments
Post a Comment